Catalyst Q&A Series – Part 6. Catalyst vs. Catalysis: What’s the Difference?
2026.01.30
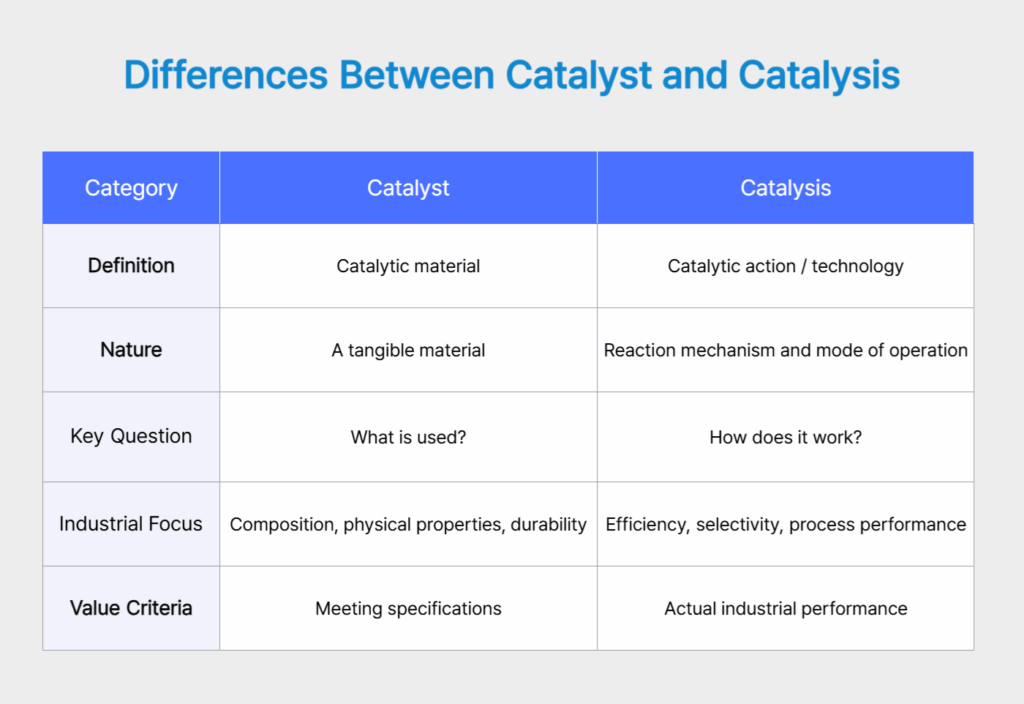
In the catalyst industry, the terms Catalyst and Catalysis are often used together. While they may appear similar, they represent clearly different perspectives on catalytic technology. Catalyst refers to the material itself, whereas Catalysis encompasses how that catalyst functions within a reaction and what outcomes it produces. In other words, a catalyst is the starting point, while catalysis describes the process through which a catalyst delivers results in real industrial settings.
Today’s chemical, energy, and environmental industries face simultaneous demands for improved process efficiency, reduced energy costs, compliance with environmental regulations, and long-term operational stability. Rather than focusing solely on production volume expansion or cost reduction as in the past, industrial competitiveness is now defined by the ability to maintain high efficiency and performance under increasingly stringent environmental standards while ensuring stable operations. As diverse requirements converge in a rapidly changing industrial environment, catalysts have evolved beyond materials into technologies that directly drive process performance. At the center of this evolution lies catalytic technology—catalysis.
The Importance of Catalytic Technology: A Core Solution to Process Challenges
According to a recent global catalyst market outlook report by Industry Research, more than 90% of chemical manufacturing processes worldwide use catalysts in at least one stage of production. This structural characteristic is observed not only in refining and petrochemicals, but also across the automotive, semiconductor, energy, and environmental industries.
By controlling reaction rates and pathways, catalysts create layered value. They reduce energy consumption, improve process efficiency and selectivity, ensure product quality stability, and enable compliance with environmental regulations. As a result, catalytic technology is no longer a supplementary element of individual processes but a core technology that determines overall industrial performance.
Catalytic Technology as a Driver of Industrial Transformation
Catalytic technology has served as a critical technical foundation throughout major industrial transitions. In the early 20th century, the Haber–Bosch process enabled large-scale ammonia synthesis through catalysis, expanding agricultural productivity and transforming global food supply systems. In the 1950s and 1960s, Ziegler–Natta catalysts made mass production of polyethylene and polypropylene possible, accelerating the growth of the plastics industry and shaping the foundation of modern materials industries.
In refining and petrochemicals, catalytic technologies have also played a central role in industrial transformation. Fluid catalytic cracking (FCC) and hydrocracking catalysts enabled more efficient utilization of heavy oils, improving energy efficiency and economic viability. Later, automotive emission control catalysts provided the technological basis for sustaining industrial operations under increasingly strict environmental regulations. Across these transitions, catalytic technology has consistently addressed both process performance and environmental requirements.

The Expanding Role of Catalysts in the Era of Sustainability
Stronger environmental regulations and carbon neutrality goals are further expanding the role of catalytic technology. Industries now require precise reaction control technologies that deliver high selectivity and durability even at lower operating temperatures, enabling simultaneous reductions in energy consumption and carbon emissions.
In this context, catalysts have moved beyond the role of reaction-promoting materials to become integrated technologies that consider process design, performance realization, and carbon reduction together. Moreover, catalytic technology has become an indispensable element in key pathways of the energy transition, including hydrogen production, CO₂ conversion, and biofuel synthesis.
Heesung Catalysts as a Solution Provider Across the Entire Value Chain
Heesung Catalysts does not view catalysts merely as materials introduced into processes. Instead, we places the highest value on catalysis—how catalysts operate within customer processes and what results they deliver. This approach begins with process analysis and reaction mechanism evaluation and extends through catalyst composition and structural design, as well as post-application performance assessment and continuous improvement.
By doing so, Heesung Catalysts goes beyond supplying catalysts to jointly defining and solving process challenges with customers. Starting from the catalyst itself and working together to complete catalysis, we designs solutions that consider both process efficiency and environmental performance. Through catalysis-driven innovation, Heesung Catalysts continues to support sustainable industrial transformation.
➡️ Related Reads
🔗 Catalyst Q&A Series – Part 1. Why Are Catalysts Important?
🔗 Catalyst Q&A Series – Part 2. The Role of Catalysts in a Carbon-Neutral, Hydrogen-Powered Future
🔗 Catalyst Q&A Series – Part 3. Criteria for Selecting Industrial Catalysts
🔗 Catalyst Q&A Series – Part 4. Applications of Catalysts in Major Industries (1)
🔗 Catalyst Q&A Series – Part 5. Applications of Catalysts in Major Industries (2)