Catalyst Q&A Series – Part 1: Why Are Catalysts Important?
2025.06.30

🔎 Catalysts are essential technologies that accelerate chemical reactions and reduce energy consumption.
This Q&A series is designed to help you better understand the role of catalysts, which are key to improving process efficiency and environmental performance across industries.
In this first episode, we explore what catalysts are and how they work, including their role in chemical reactions and the concept of activation energy. We also look at the differences between metal and non-metal catalysts, how automotive three-way catalysts operate, and why precious metal catalysts are reused.
Real-world examples show what could go wrong in industrial processes without catalysts and explain how catalysts improve both productivity and environmental outcomes.
1. What is a catalyst?
A catalyst is a substance that increases the rate of a chemical reaction. Simply put, it acts as a “reaction accelerator” by temporarily interacting with reactants to create a more efficient reaction pathway, thereby lowering the activation energy required. Catalysts are not consumed in the reaction and remain unchanged after the process, making them essential for improving process efficiency and reducing energy consumption.
2. How do catalysts affect chemical reactions?
Catalysts make chemical reactions proceed more easily by lowering the activation energy, which is the energy barrier that must be overcome for a reaction to occur. With the help of catalysts, reactions that once required high temperatures and pressures can now take place under milder conditions. This leads to increased productivity and reduced costs in manufacturing processes. As reaction rates rise, process times shorten, and overall plant efficiency improves significantly.
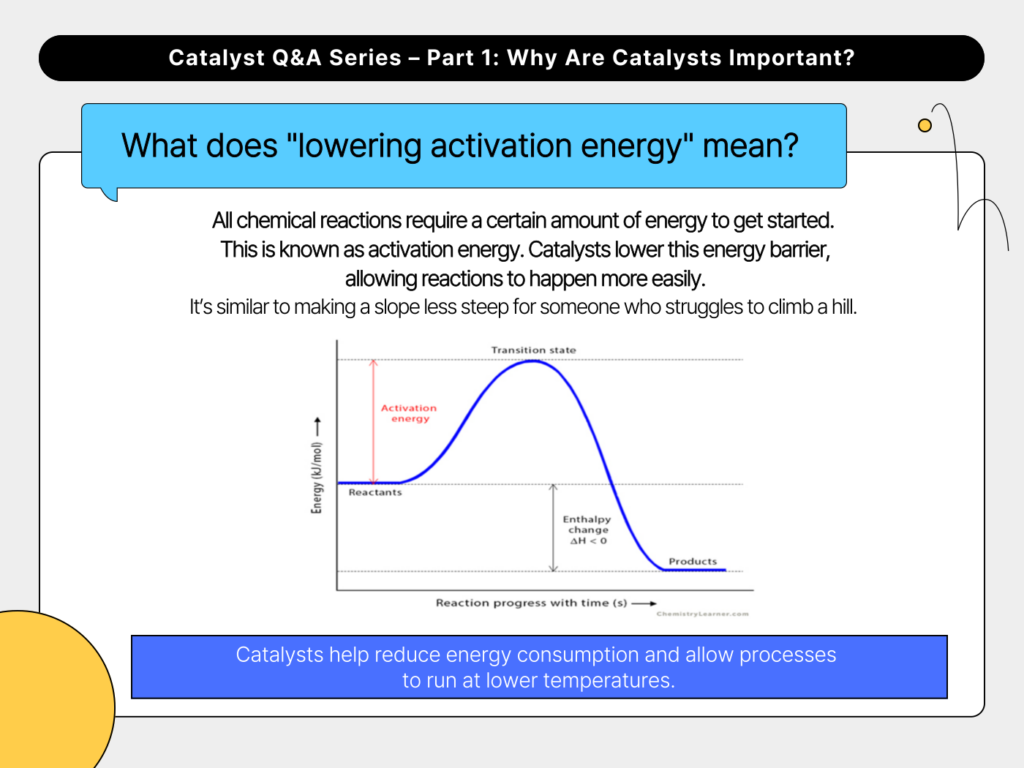
3. What does “lowering activation energy” mean?
All chemical reactions require a certain amount of energy to get started. This is known as activation energy. Catalysts lower this energy barrier, allowing reactions to happen more easily. It’s similar to making a slope less steep for someone who struggles to climb a hill. By doing so, catalysts help reduce energy consumption and allow processes to run at lower temperatures.
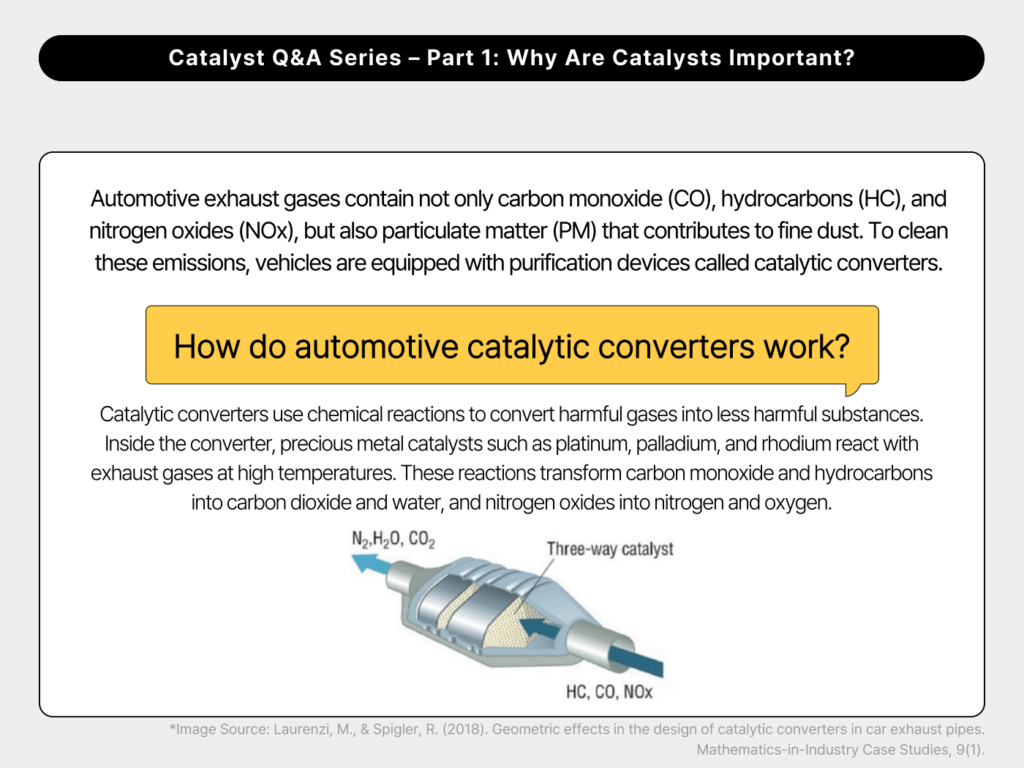
4. How do automotive catalytic converters work?
Automotive exhaust gases contain not only carbon monoxide (CO), hydrocarbons (HC), and nitrogen oxides (NOx), but also particulate matter (PM) that contributes to fine dust. To clean these emissions, vehicles are equipped with purification devices called catalytic converters.
Catalytic converters use chemical reactions to convert harmful gases into less harmful substances. Inside the converter, precious metal catalysts such as platinum, palladium, and rhodium react with exhaust gases at high temperatures. These reactions transform carbon monoxide and hydrocarbons into carbon dioxide and water, and nitrogen oxides into nitrogen and oxygen.
Among various technologies, the Three-Way Catalyst (TWC) is especially notable. It removes all three major pollutants, including CO, HC, and NOx, in a single unit. This technology plays a key role in meeting global automotive emission standards and helps reduce emissions without sacrificing fuel efficiency or engine performance.
✅ Beyond regulations, towards sustainable mobility with Heesung Catalysts
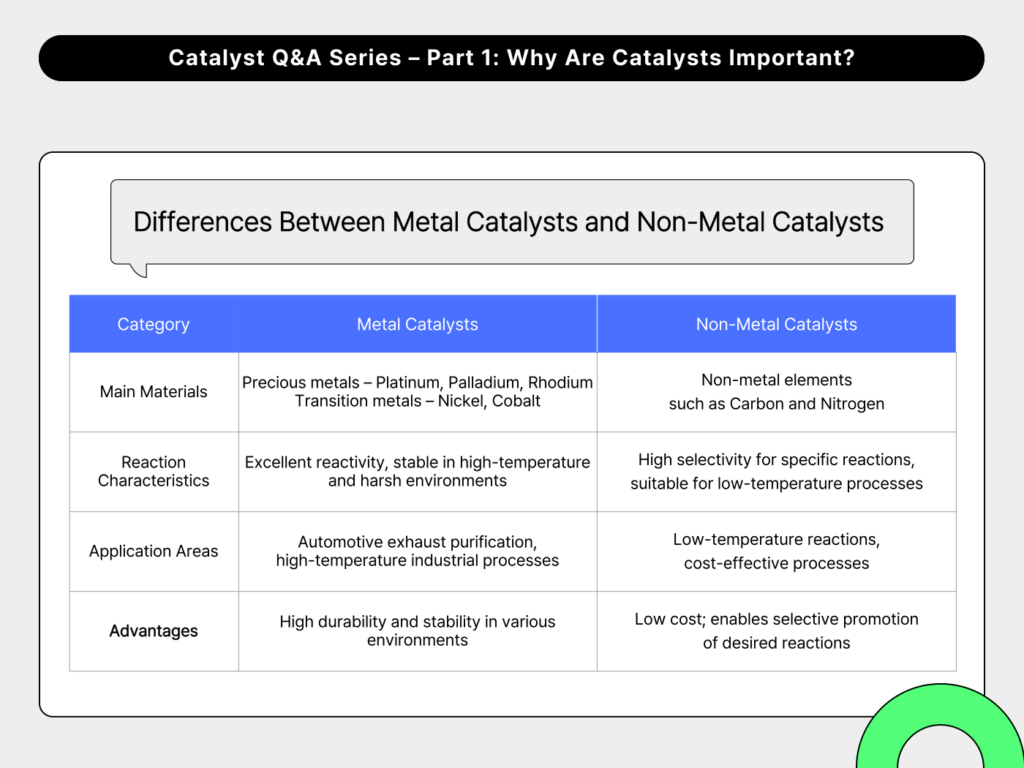
5. What is the difference between metal catalysts and non-metal catalysts?
Catalysts can be classified into metal and non-metal types based on their elemental composition, and the two differ in both material properties and reaction behavior.
Metal catalysts are typically made from precious metals such as platinum (Pt), palladium (Pd), and rhodium (Rh), or transition metals like nickel (Ni) and cobalt (Co). These catalysts exhibit high reactivity and remain stable even under high-temperature and high-pressure conditions. For this reason, they are commonly used in processes that require fast and intense reactions, such as automotive exhaust purification and high-temperature industrial applications.
Non-metal catalysts, on the other hand, are made from elements like carbon and silicon. They are generally used in lower-temperature processes or in applications where high selectivity is required—reacting only with specific substances. Because they are often less expensive than metal catalysts, they are also preferred in cost-sensitive applications.
6. How are precious metal catalysts recycled?
Even after use, precious metal catalysts still contain valuable metal components. Instead of being discarded, they are collected and sent to specialized facilities where the metals are extracted, purified, and reused as resources.
Heesung Catalysts manages this entire resource recovery process in a systematic way. From collecting spent catalysts to extracting and purifying metals and producing new catalysts, we ensure strict quality control and minimize environmental impact at every stage. This approach helps reduce resource waste and supports our customers in improving process efficiency and achieving their ESG goals.
In recent years, as industries such as manufacturing and energy place greater emphasis on carbon neutrality and resource security, the recovery and recycling of not only precious metals but also transition metals and critical minerals like nickel and cobalt have become increasingly important.
✅ Innovative catalyst subscription service for a circular economy
7. What problems would arise in industrial processes without catalysts?
Without catalysts, industrial processes would require higher temperatures and pressures, as well as longer reaction times. This would significantly increase energy consumption and operational costs, while also leading to greater greenhouse gas emissions. In industries such as chemicals, refining, automotive, and semiconductors, maintaining the desired level of quality and productivity would be extremely challenging without the use of catalysts.
Heesung Catalysts offers a wide range of catalyst solutions that help customers improve productivity and energy efficiency across various industrial processes, while also supporting compliance with environmental regulations.
✅ Innovative reaction process solutions make industry more efficient and sustainable
➡️ Read More